DESCRIPTION:
The Device is intended to be used for denudation i.e. to remove cumulus oocytes complexes from oocytes before procedure,”Intracytoplasmic Sperm Injection (ICSI)” and handling or manipulating of oocytes and embryos in Assisted reproduction technique (ART).
FEATURES:
- Designed to adapt to surfaces with flexibility, lowering the risk of scratching or breaking.
- Precisely engineered for delicate and accurate handling.
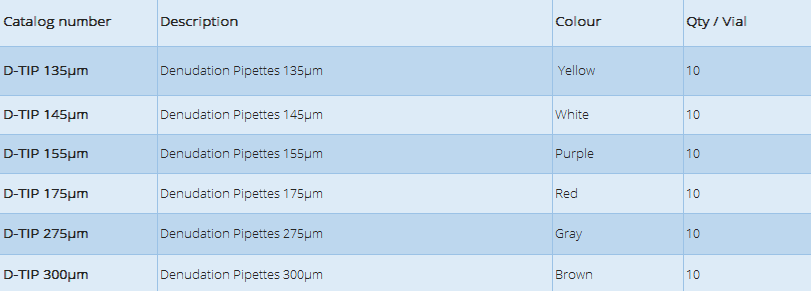
- Different Color code for different sizes of pipette.
- Endotoxin Tested (LAL test) 20EU/device.
- Mouse Embryo Assay (MEA) ≥ 80%.
- Gamma Sterilized.
- Mouse embryo assay tested (MEA).
- Limulus amebocyte lysate tested (LAL).